Reduction of 75 and 77 percent seen in estimated mean monthly attack rate with 25-mg, 50-mg NTLA-2002 versus placebo
By Elana Gotkine HealthDay Reporter
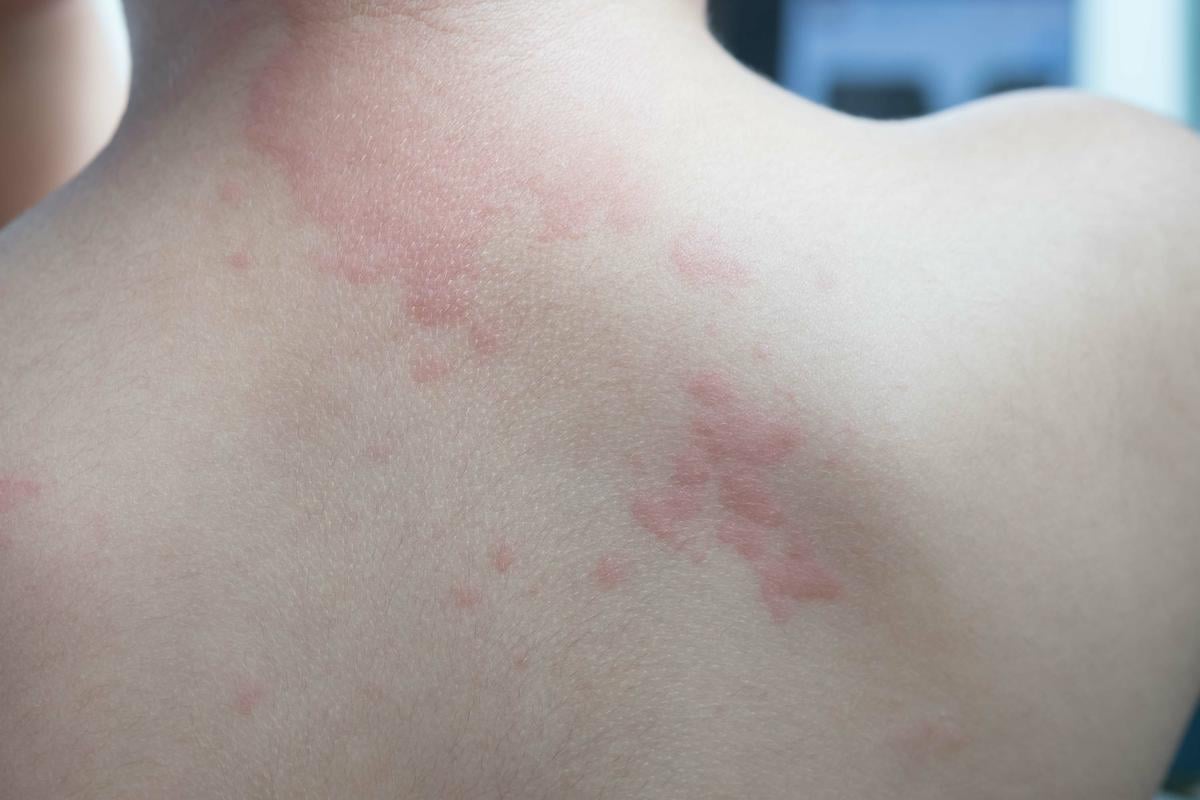
THURSDAY, Oct. 24, 2024 (HealthDay News) — For adults with hereditary angioedema, the in vivo gene-editing therapy NTLA-2002, which is based on clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated protein 9, administered as a single dose reduces angioedema attacks, according to a study published online Oct. 24 in the New England Journal of Medicine to coincide with the annual meeting of the American College of Allergy, Asthma & Immunology, held from Oct. 24 to 28 in Boston.
Danny M. Cohn, M.D., Ph.D., from the Amsterdam University Medical Center, and colleagues randomly assigned adults with hereditary angioedema to receive NTLA-2002 in a single dose of 25 mg or 50 mg or placebo in a 2:2:1 ratio (10, 11, and six patients, respectively). The number of angioedema attacks per month from week 1 to 16 was the primary end point.
The researchers found that the estimated mean monthly attack rate was 0.70, 0.65, and 2.82 with 25-mg NTLA-2002, 50-mg NTLA-2002, and placebo, respectively (differences, −75 and −77 percent, respectively, compared with placebo). Overall, 40 and 73 percent of the patients who received 25-mg and 50-mg NTLA-2002, respectively, were attack-free with no additional treatment during the period from weeks 1 to 16. Headache, fatigue, and nasopharyngitis were the most common adverse events among patients receiving NTLA-2002.
“These results show the potential of a single dose of the new CRISPR-Cas9-based in vivo gene editing therapy NTLA-2002 to be a functional cure for patients with hereditary angioedema and support continued investigation in a larger phase 3 trial,” the authors write.
The study was funded by Intellia Therapeutics, which is developing NTLA-2002.
Editorial (subscription or payment may be required)
Copyright © 2024 HealthDay. All rights reserved.