Approval is for adults with moderately to severely active disease
By Lori Solomon HealthDay Reporter
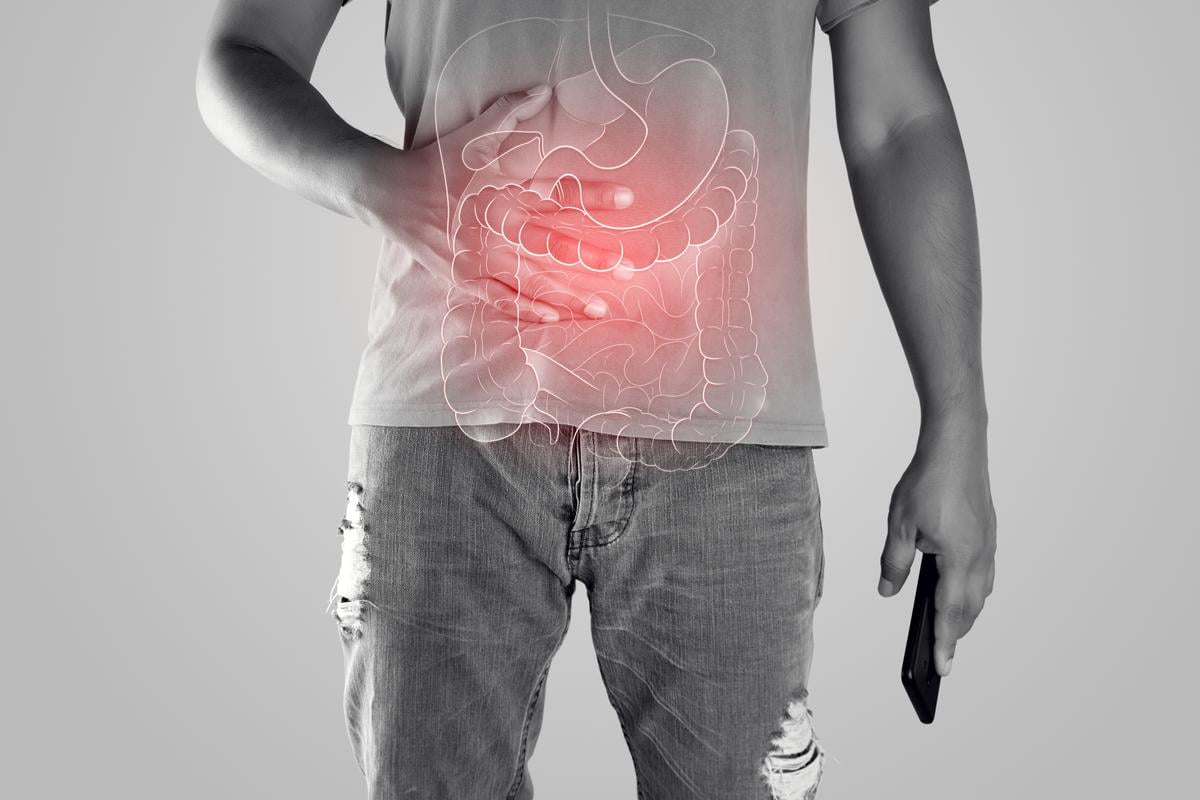
MONDAY, Sept. 16, 2024 (HealthDay News) — The U.S. Food and Drug Administration has approved Tremfya (guselkumab) for the treatment of adults with moderately to severely active ulcerative colitis.
Tremfya is the first and only dual-acting interleukin-23 inhibitor approved in active ulcerative colitis. It is approved for administration as a 200-mg induction dose intravenously at weeks 0, 4, and 8 by a health care professional.
The approval is based on results from the phase 2b/3 QUASAR study. Half of patients receiving Tremfya 200 mg subcutaneous (SC) maintenance every four weeks and 45 percent of patients receiving Tremfya 100 mg SC every eight weeks achieved the primary end point of clinical remission at week 44 versus 19 percent of patients receiving placebo. At one year, 34 percent (200 mg) and 35 percent (100 mg) of patients achieved endoscopic remission compared with15 percent receiving placebo.
“Treatment with Tremfya resulted in significant improvement in the chronic symptoms of ulcerative colitis, and importantly, normalization in the endoscopic appearance of the intestinal lining,” David T. Rubin, M.D., from the University of Chicago Medicine and lead investigator for the QUASAR program, said in a statement.
Approval of Tremfya was granted to Johnson & Johnson.
Copyright © 2024 HealthDay. All rights reserved.