Pharmedica and Apotex voluntarily recalling products due to issues with sterility
By Physician’s Briefing Staff HealthDay Reporter
TUESDAY, March 7, 2023 (HealthDay News) — U.S. federal health officials have issued recall notices for two more brands of eye drops.
In the latest round of recalls, the U.S. Food and Drug Administration posted notices after the companies voluntarily pulled several lots of their eye drops from the market.
The companies involved in the recalls are Phoenix-based Pharmedica and Florida-based Apotex.
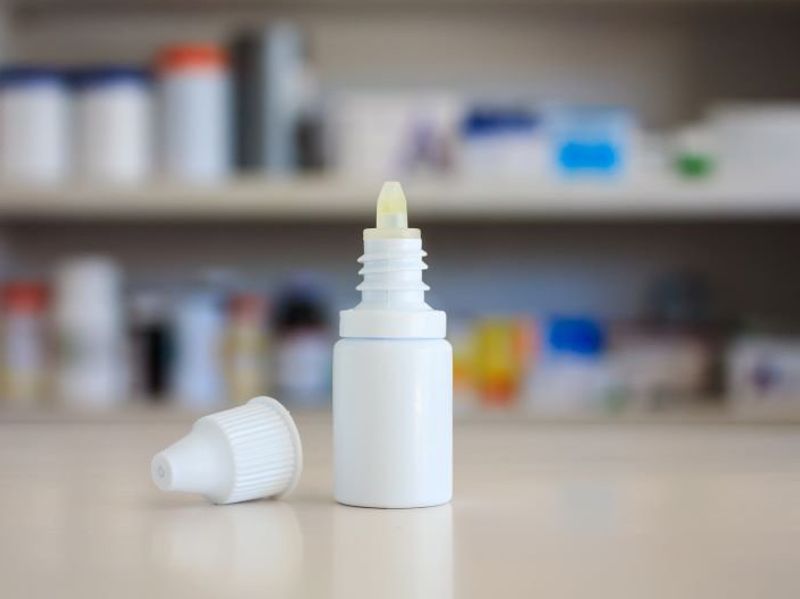
Pharmedica is recalling its Purely Soothing 15 percent MSM Drops meant to treat eye irritation. The two lots were pulled because of “nonsterility” and “risk of eye infections that could result in blindness,” the company said. People who have the eye drops should immediately stop using them and return them to the store where they bought them, the company added.
Meanwhile, Apotex is recalling six lots of prescription eye drops distributed as Brimonidine Tartrate Ophthalmic Solution, 0.15 percent. They were sold between April 2022 and February 2023. These eye drops are meant to treat glaucoma. Unfortunately, some of the eye drop bottles have cracks in the caps, the company said.
Recall Notice – Pharmedica
Recall Notice – Apotex
Copyright © 2023 HealthDay. All rights reserved.