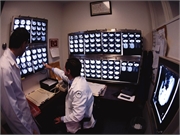
Most patients had negative PET scan and completed one additional R-CHOP cycle without radiation
MONDAY, Dec. 9, 2019 (HealthDay News) — Positron emission tomography (PET)-directed therapy results in excellent outcomes for patients with stage I/II diffuse large B-cell lymphoma (DLBCL), according to a study presented at the annual meeting of the American Society of Hematology, held from Dec. 7 to 10 in Orlando, Florida.
Daniel O. Persky, M.D., from the University of Arizona in Tucson, and colleagues designed a PET-directed study to tailor therapy after three cycles of rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for patients with non-bulky stage I/II untreated DLBCL. Patients with a negative scan proceeded with an additional cycle of R-CHOP, while those with a positive PET scan initiated involved field radiation therapy (IFRT) plus an additional boost. This was followed by ibritumomab tiuxetan (IFRT-Zevalin) three to six weeks after completing IFRT. Data were included for 132 eligible patients including 128 with an interim PET scan.
The researchers found that 110 of the patients with a PET scan were iPET-negative. Of the 14 true iPET-positive patients, 12 received IFRT-Zevalin. Sixty-seven percent converted from partial response to complete response after IFRT-Zevalin and 33 percent had partial response. Only five patients progressed, and two died from lymphoma within a median follow-up of 4.5 years. The five-year progression-free survival (PFS) estimate was 87 percent and the overall survival (OS) estimate was 90 percent; similar outcomes were seen for iPET-positive and iPET-negative patients (PFS: 86 versus 88 percent; OS: 93 versus 91 percent).
“We find that many patients can forgo radiation and get less chemo — and still get excellent results,” Persky said in a statement.
Several authors disclosed financial ties to the pharmaceutical industry; the study was partly funded by Spectrum Pharmaceuticals.
Copyright © 2019 HealthDay. All rights reserved.