The nonsteroidal topical treatment is approved for patients 2 years and older
By Lori Solomon HealthDay Reporter
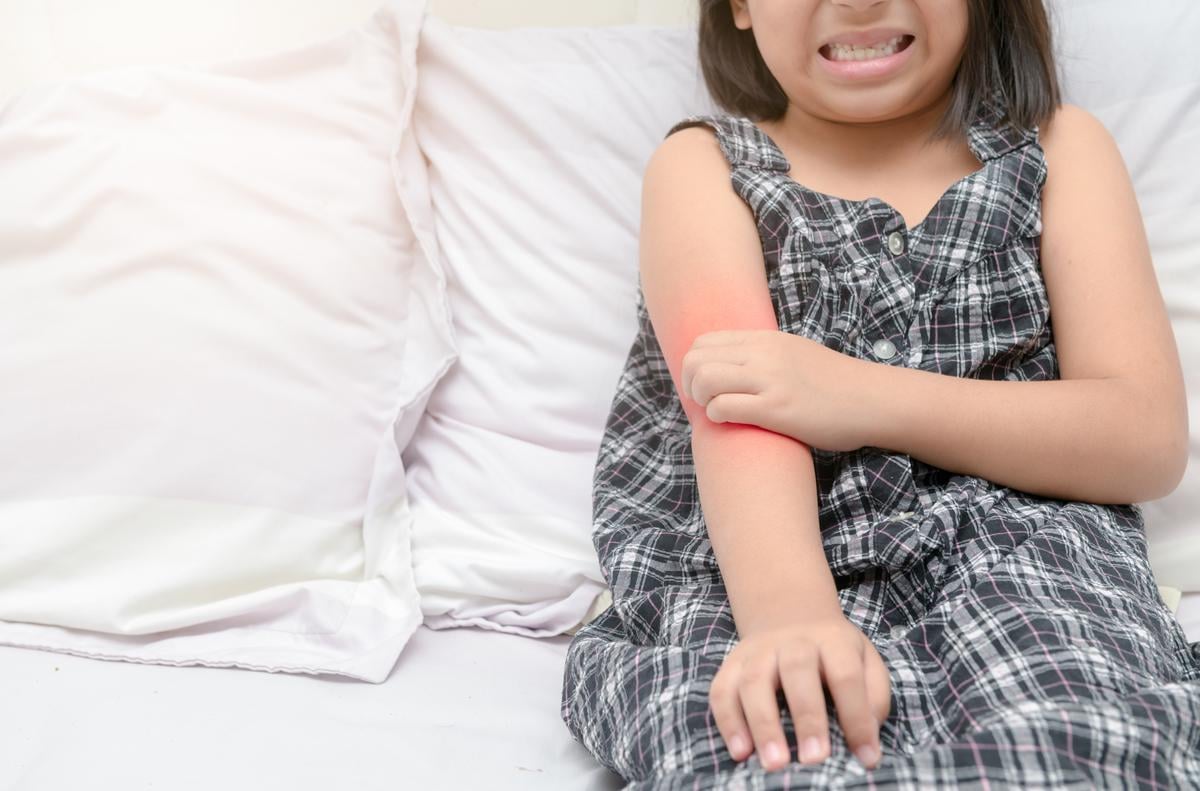
THURSDAY, Dec. 19, 2024 (HealthDay News) — The U.S. Food and Drug Administration has approved Vtama (tapinarof) 1 percent cream for an additional indication — the topical treatment of atopic dermatitis in adult and pediatric patients aged 2 years and older.
The cream, an aryl hydrocarbon receptor agonist, was previously approved as a topical, nonsteroidal treatment for plaque psoriasis.
The approval was based on results from the ADORING 1 and ADORING 2 trials, in which a statistically significant difference in the proportion of patients achieving a score of clear (0) or almost clear (1) and a minimum two-grade improvement from baseline at week 8 on the Validated Investigator Global Assessment for AD (vIGA-AD) was seen for Vtama compared with vehicle (ADORING 1: 45.4 versus 13.9 percent; ADORING 2: 46.4 versus 18.0 percent). Statistically significant benefits were seen for secondary end points, including an Eczema Area and Severity Index score improvement of at least 75 percent from baseline at week 8 and achievement of a ≥4-point improvement in the patient-reported Peak Pruritus Numerical Rating Scale from baseline at week 8 in patients aged 12 years and older.
Upper respiratory tract infection (12 percent), folliculitis (9 percent), lower respiratory tract infection (5 percent), headache (4 percent), asthma (2 percent), vomiting (2 percent), ear infection (2 percent), pain in extremity (2 percent), and abdominal pain (1 percent) were the most common adverse reactions reported.
“With the FDA’s approval of Vtama cream in atopic dermatitis for adults and children as young as 2 years old, there is now a therapy that offers the potential for powerful skin clearance with no label warnings or precautions, contraindications, and no restrictions on duration of use or percentage of body surface area affected,” Kevin Ali, the CEO of Organon, said in a statement.
Approval of Vtama was granted to Organon.
Copyright © 2024 HealthDay. All rights reserved.