In phase 2 randomized trial, 2.5 ml less brain-tissue loss with ibudilast than with placebo over 96 weeks
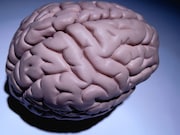
FRIDAY, Aug. 31, 2018 (HealthDay News) — In patients with progressive multiple sclerosis, slower progression of brain atrophy was seen with ibudilast versus placebo, according to a phase 2 trial published in the Aug. 30 issue of the New England Journal of Medicine.
Robert J. Fox, M.D., from the Cleveland Clinic, and colleagues conducted a phase 2 trial in which patients with primary or secondary progressive multiple sclerosis were randomized to ≤100 mg daily oral ibudilast (129 participants) or placebo (126 participants) for 96 weeks. A total of 53 percent of the patients in the ibudilast group and 52 percent of those in the placebo group had primary progressive disease, while the others had secondary progressive disease.
The researchers found that the rate of change in the brain parenchymal fraction was −0.001 per year with ibudilast and −0.0019 per year with placebo, which represents approximately 2.5 ml less brain-tissue loss with ibudilast over the study period. Adverse events with ibudilast included gastrointestinal symptoms, headache, and depression.
“Further trials are needed to identify whether the effect on brain atrophy is reproducible and is associated with slowed progression of neurologic disability,” the authors write.
Several authors disclosed financial ties to pharmaceutical companies, including MediciNova, which manufactures ibudilast and provided funding for the study.
Abstract/Full Text (subscription or payment may be required)
Copyright © 2018 HealthDay. All rights reserved.